Lung Cancer Treatment Guide: Latest Strategies, Statistics, and Drug Developments
"A journey of a thousand miles begins with a single step." — Laozi
What Is Lung Cancer?
Lung cancer, medically referred to as primary bronchogenic carcinoma, is the most common malignant tumor originating from the epithelial cells of the trachea, bronchi, or lung glands. It is among the leading causes of cancer-related deaths worldwide, including in China.
Lung cancer is broadly classified into two main types:
- Non-Small Cell Lung Cancer (NSCLC): Makes up approximately 80–85% of cases. Subtypes include adenocarcinoma, squamous cell carcinoma, adenosquamous carcinoma, and large cell carcinoma.
- Small Cell Lung Cancer (SCLC): Less common but more aggressive, often requiring a combination of chemotherapy and radiotherapy.
From a genomic standpoint, common mutations in NSCLC include EGFR, ALK, ROS1, BRAF, among others. Treatment varies greatly depending on the cancer subtype, genetic mutation profile, and clinical stage.
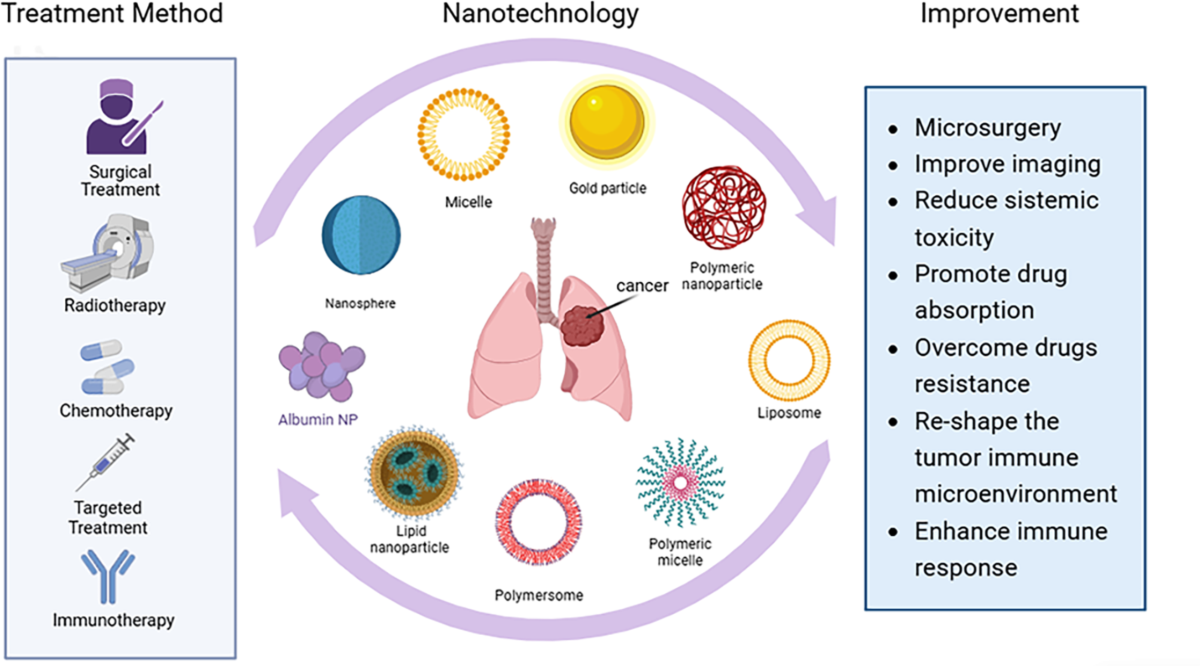
Global and Chinese Lung Cancer Statistics (2024)
On April 4, 2024, CA: A Cancer Journal for Clinicians (Impact Factor: 254.7) released its latest global cancer statistics:
- 1 in 5 people globally will develop cancer in their lifetime.
- 1 in 9 men and 1 in 12 women will die from cancer.
- In 2022, an estimated 19.96 million new cancer cases and 9.74 million cancer deaths occurred worldwide.
Lung Cancer Snapshot:
- #1 most diagnosed cancer globally (12.4% of all new cases).
- #1 cause of cancer-related deaths (18.7% of all cancer deaths).
- In China, 2022 saw 1.06 million new lung cancer cases.
- 5-year survival rate for late-stage lung cancer remains around 20%.
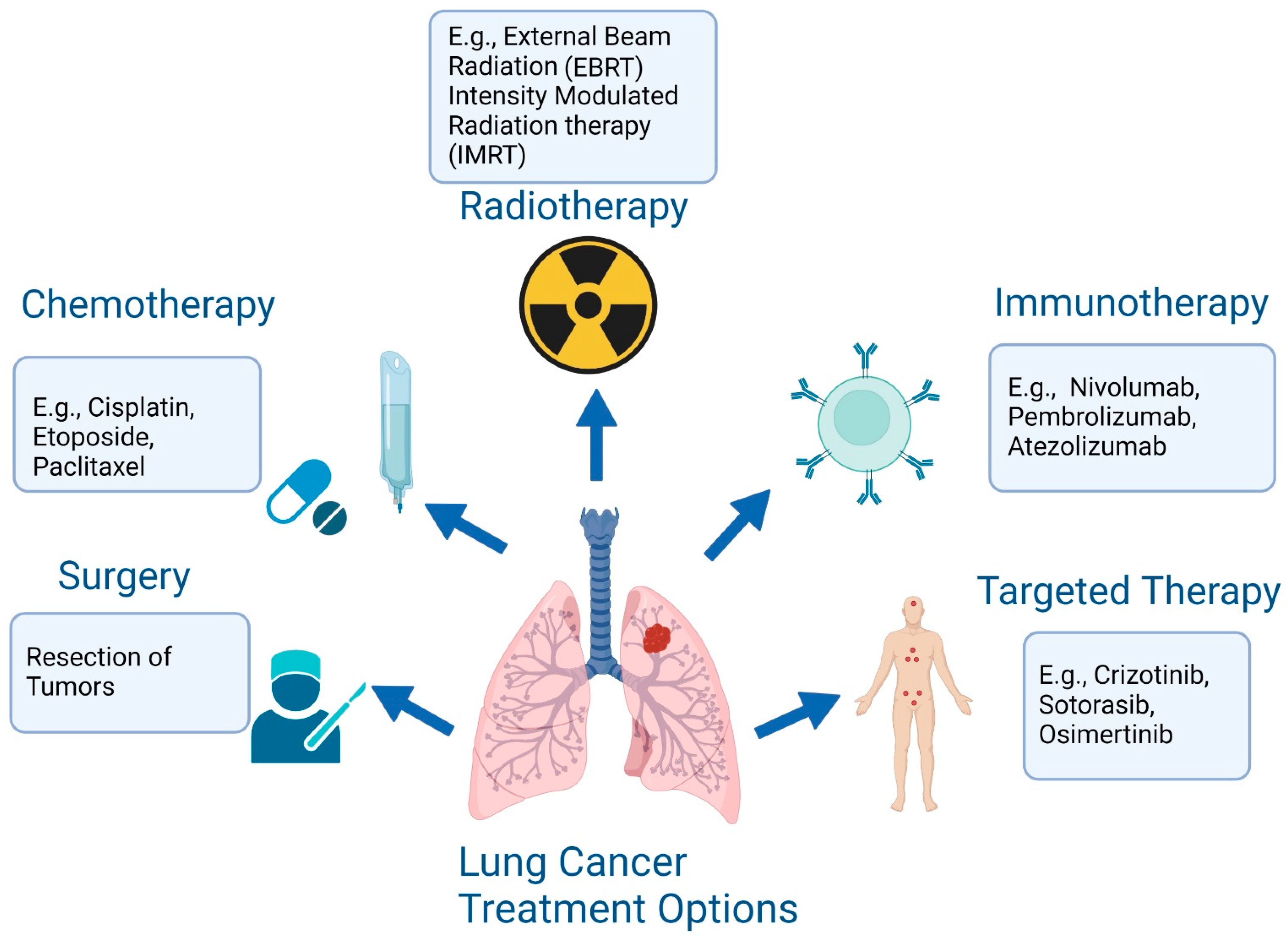
Stage-Based Treatment Approaches
Stage I–II NSCLC
For early-stage NSCLC, the gold standard is surgical resection, including anatomical lobectomy and mediastinal lymph node dissection. Surgery offers the best chance for long-term survival when diagnosed early.
Stage III NSCLC
Treatment decisions in stage III depend on resectability:
- Resectable cases (e.g., T3N1, T4N0-1, select T1-2N2): Surgical resection is part of a multidisciplinary approach.
- Requires comprehensive evaluation by a multidisciplinary team (MDT) to determine surgical eligibility and the best combination with chemotherapy or radiotherapy.
Stage IV NSCLC: Systemic and Targeted Therapies
1. Patients with Driver Mutations (Non-squamous NSCLC)
- EGFR mutations: Osimertinib, Aumolertinib, Furmonertinib, Gefitinib, Erlotinib, Icotinib, Afatinib, Dacomitinib
- ALK rearrangement: Lorlatinib, Ensartinib, Alectinib, Ceritinib, Brigatinib, Crizotinib
- ROS1 rearrangement: Crizotinib, Entrectinib
- MET exon 14 skipping: Savolitinib, Glumetinib
- BRAF V600E mutation: Dabrafenib + Trametinib
- RET rearrangement: Selpercatinib
Patients without mutations or who progress after targeted therapy may proceed to chemoimmunotherapy.
2. Patients Without Driver Mutations
- Performance Status (PS) 0–1: Platinum doublet chemotherapy + immune checkpoint inhibitors (e.g., pembrolizumab, toripalimab).
- PS 2: Single-agent chemotherapy (e.g., gemcitabine, vinorelbine, paclitaxel, pemetrexed).
- PS 3–4: Supportive care or clinical trials; systemic chemotherapy is not recommended.
3. Squamous NSCLC
Treatment is similar but tailored:
- No driver mutations: Carboplatin + paclitaxel + immunotherapy (e.g., tislelizumab, camrelizumab).
- Driver mutation positive: Treat similarly to non-squamous cases with targeted agents.
Targeted Therapy: Precision Oncology in Action
Genetic testing is now essential for all advanced NSCLC patients regardless of histology—especially for non-smokers or those with mixed histologic features. Genes like EGFR, ALK, ROS1, MET14, BRAF are routinely screened. Treatment is based on mutation type, resistance status, and previous lines of therapy.
China's New Drug Pipeline for Lung Cancer (2020–2024)
China has rapidly emerged as a major player in global oncology R&D. As of 2024:
- Over 2,800 small-molecule anti-cancer drugs are in development.
- NSCLC ranks among the top targets alongside breast, prostate, and colorectal cancers.
Notable Domestic Approvals:
| Drug Name | Approval Year | Company | Indication |
|---|---|---|---|
| Vebreltinib | 2023 | Crown Biosciences | NSCLC |
| Sunvozertinib | 2023 | Dizal Pharma | NSCLC |
| Iruplinalkib | 2023 | Qilu Pharma | NSCLC |
| Glumetinib | 2023 | Green Valley + CAS | MET exon 14 mutated NSCLC |
| Savolitinib | 2021 | Hutchmed | MET+ NSCLC |
| Osimertinib | 2015 | AstraZeneca | EGFR+ NSCLC |
Many of these drugs are now available in China before or simultaneously with global markets, greatly improving local access to cutting-edge therapies.
Final Thoughts
Lung cancer remains one of the most challenging malignancies globally. While advances in genomic profiling and targeted therapy have dramatically improved outcomes for certain subtypes, early detection and multidisciplinary care are still key to success.
With China's growing pipeline of approved lung cancer treatments and continued research into innovative therapies, the future of lung cancer care is promising.
Categories: health
